AARP Hearing Center
Conditions & Treatments
How Revolutionary Brain Implants Are Beginning to Restore Abilities and Combat Disease
In trials with a handful of patients, new technologies help improve movement and speech
By Michael Greshko, AARP
Published November 20, 2024
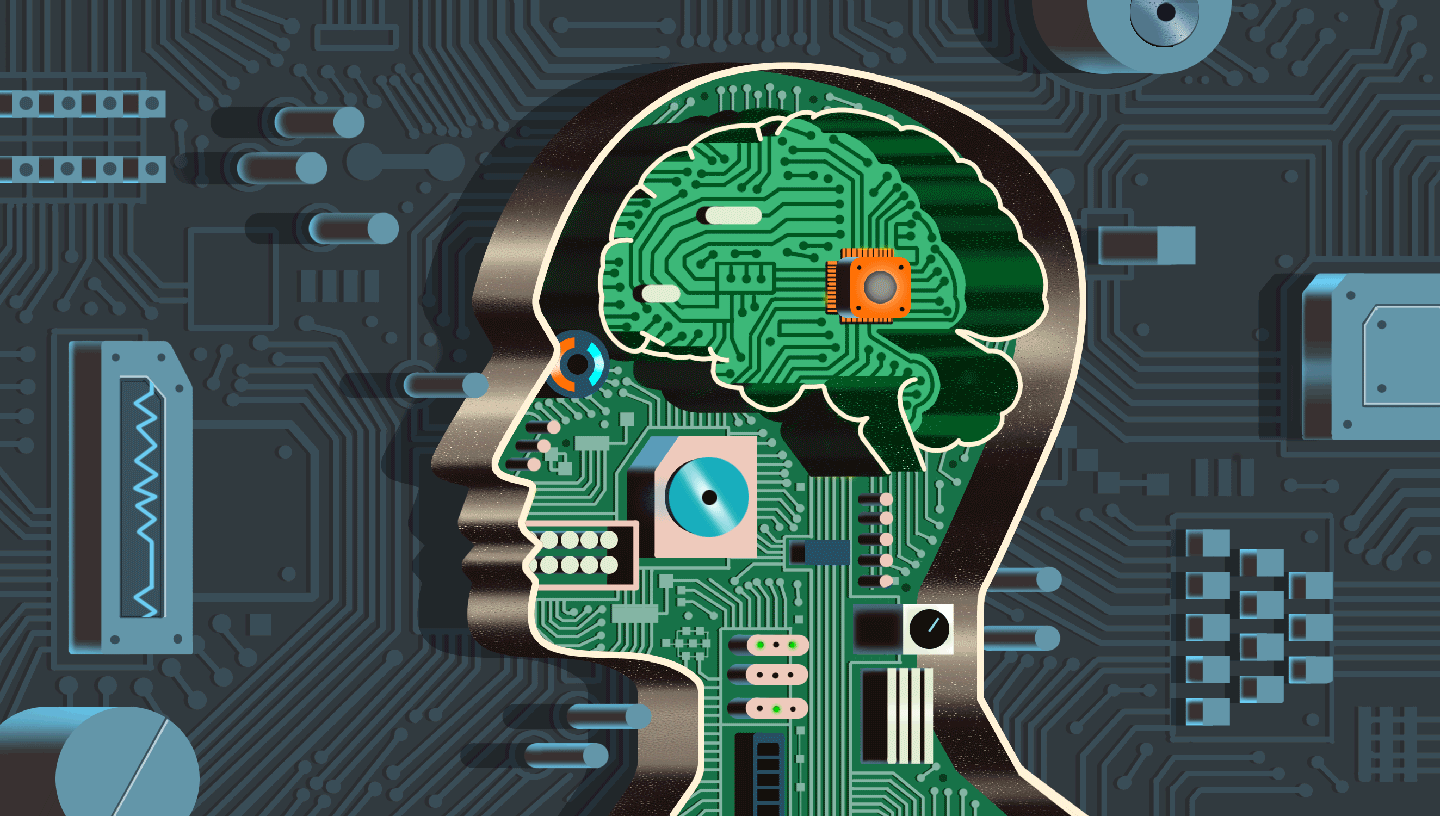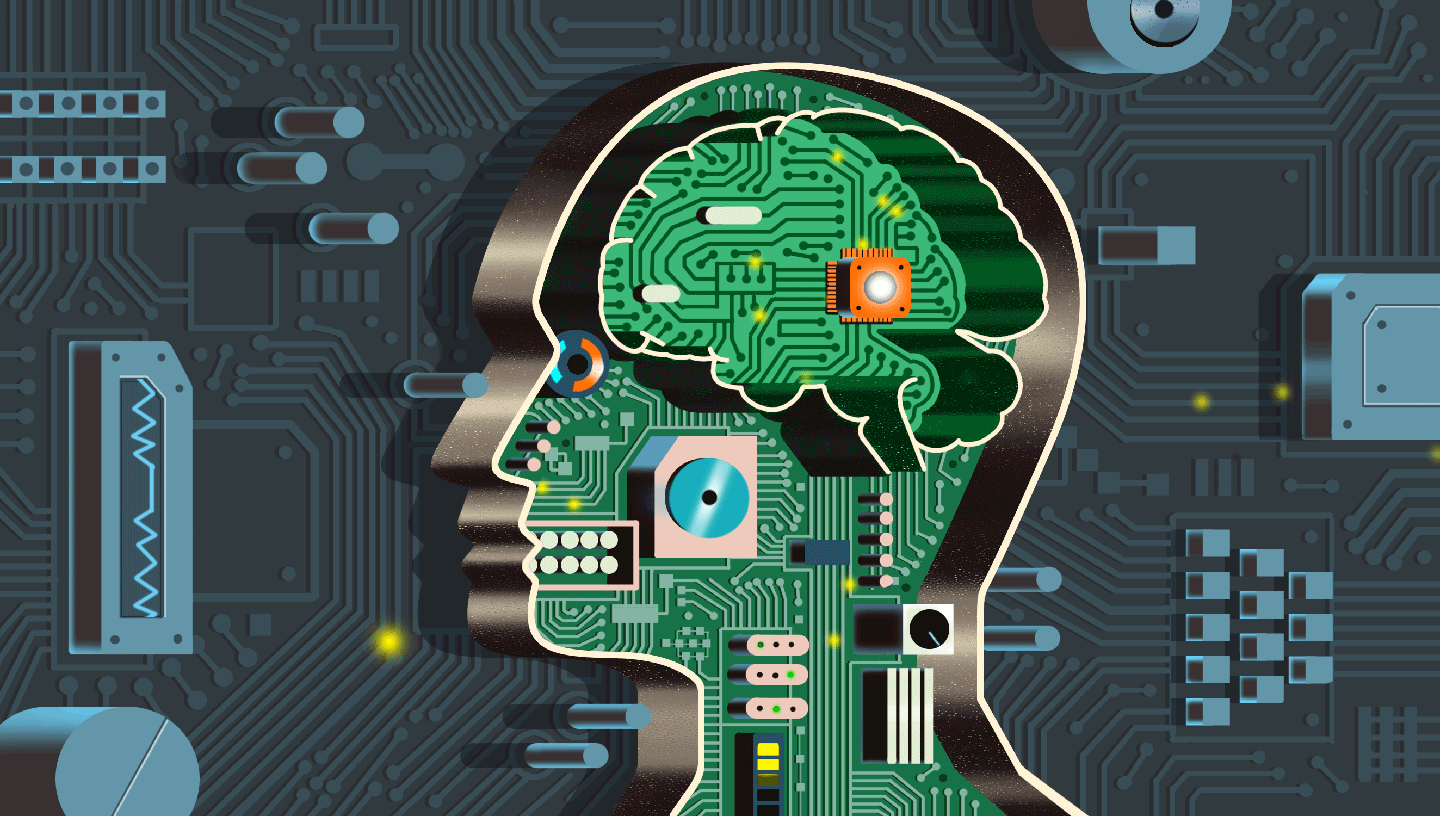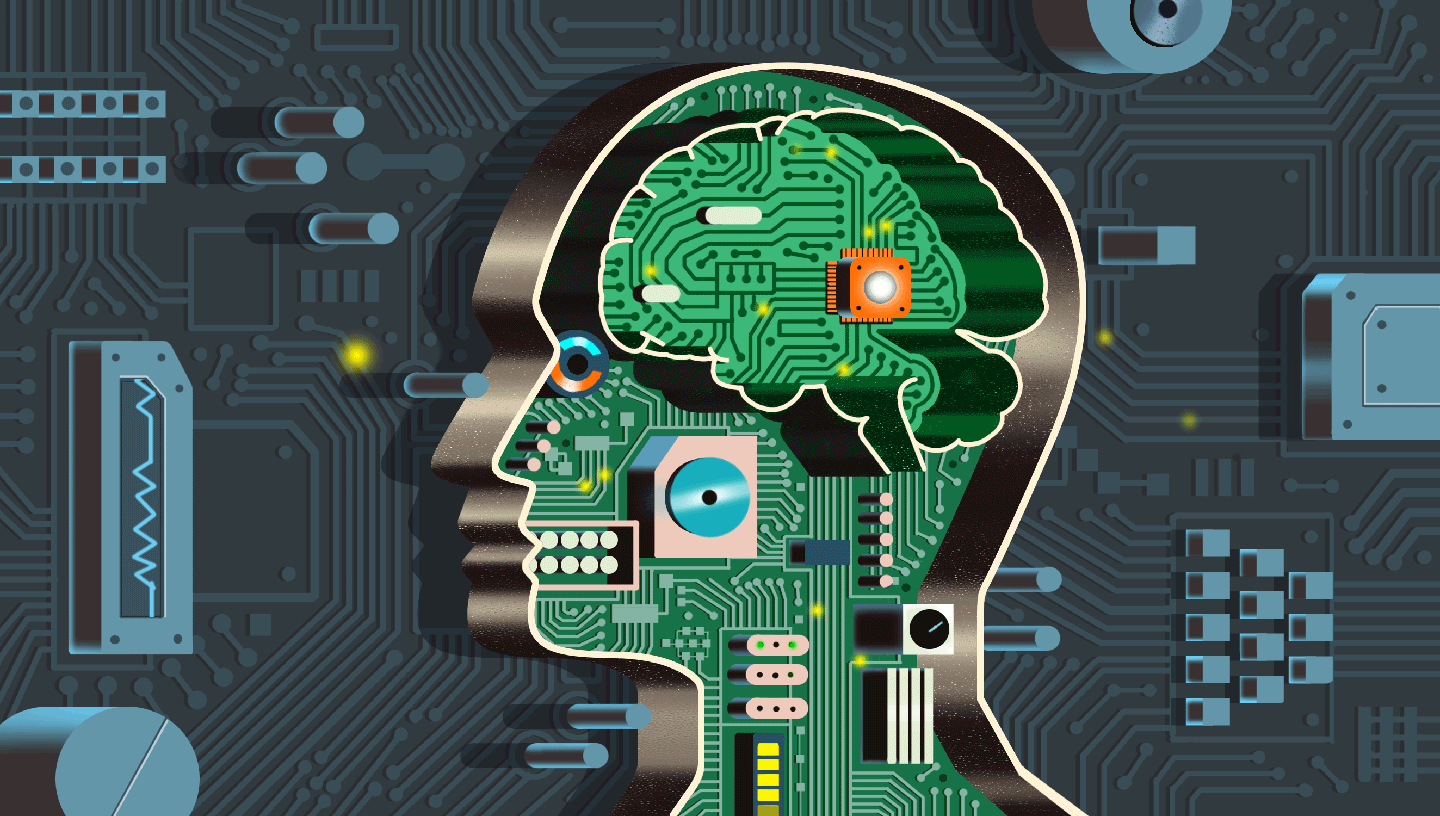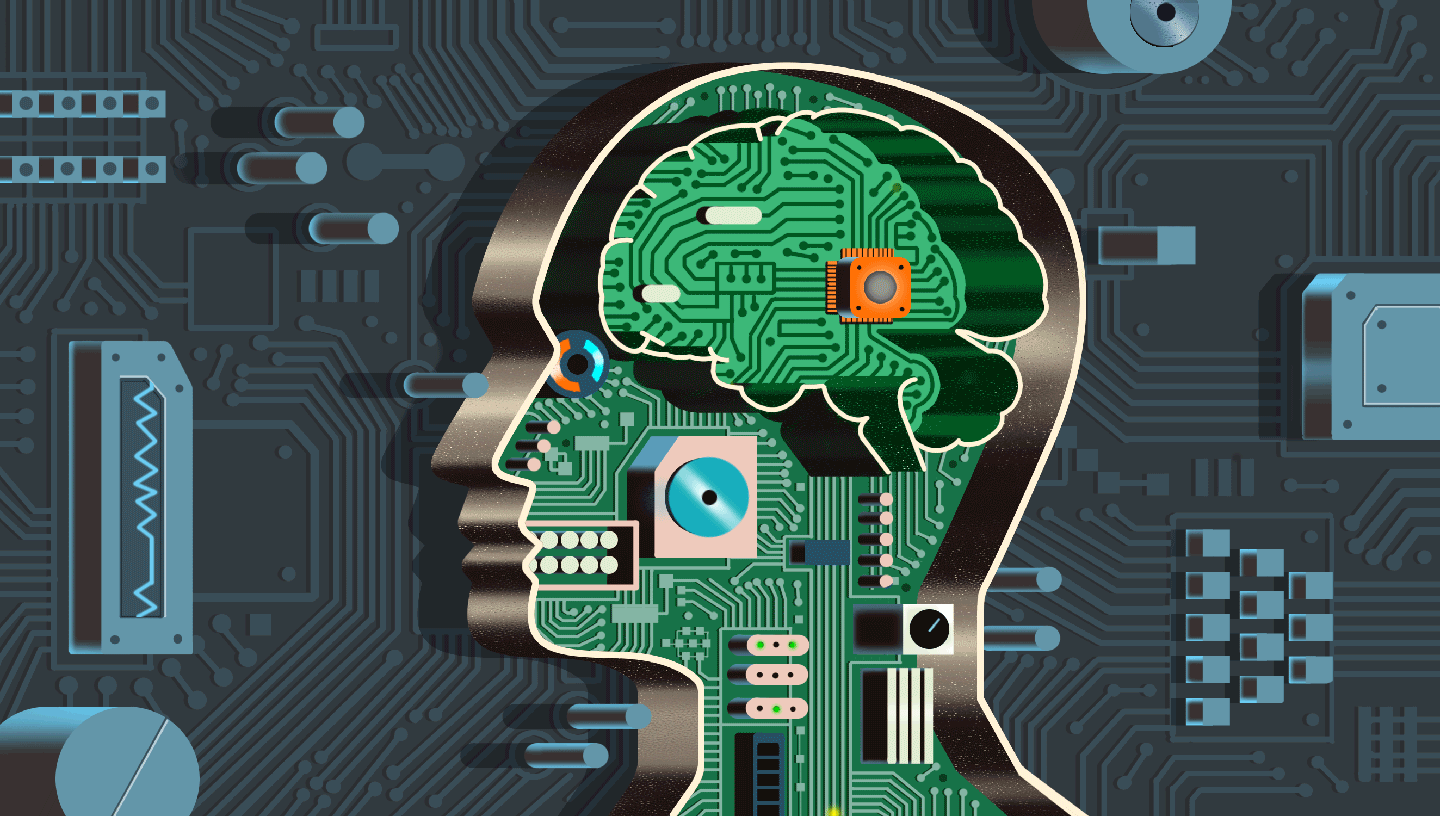
Imagine a brain chip capable of decoding thoughts and intentions in ways that could help a person speak or move again, years after losing these skills to a devastating illness or injury.
It may sound like science fiction, but in laboratories around the world, researchers are developing therapies that rely on devices called brain-computer interfaces (BCIs for short) to help address several serious health issues that affect many adults age 50-plus.
You may have heard of this idea before: A brain-implanted chip was first used to control a computer cursor nearly two decades ago, in 2006. Or you may have seen more advanced BCIs in the news recently. Tech billionaire Elon Musk’s brain-implant company Neuralink launched its first human clinical trial this year and planted a brain chip in a 30-year-old man who was paralyzed below the neck after an accident in 2016.
The device — which is about the size of a quarter and sits near the top of the skull above the ear, while its wires weave through an area of the brain that controls movement and intention — allows a person to control a computer cursor with their thoughts. The first patient in the trial, for example, was able to play digital chess and video games with the chip. A second participant who received the brain chip over the summer is learning how to use computer software to design 3D objects that are then printed.
Neuralink sees this capability as a first step toward potentially creating a seamless interface between the human brain and computers. If computers could one day decode brain activity very quickly and accurately, they could power technologies that could help restore complete autonomy to people who have lost sight, speech or movement.
“Taking an idea, putting it as a design, and actually having a physical item as a finished product makes me feel like I’m building things again,” the second trial participant said in a post on Neuralink’s website.
Neuralink is just one of many private businesses and university research labs developing new BCI technologies, and others are working to improve brain implants we already have. And though it will be years before innovations make their way through the regulatory process and into doctors’ offices, it’s still “a really exciting time for neurotechnology right now,” says David McMullen, M.D., director of the Office of Neurological and Physical Medicine Devices at the U.S. Food and Drug Administration (FDA), which oversees BCIs.
Here’s a look at what’s happening.
How we are already using — and improving — brain implants
First, know that some people are already benefiting from brain and spine implants.
Around the world, about 34,000 people per year receive spinal cord stimulators, devices that are implanted next to the spinal cord and release low-level electrical pulses that help manage types of back pain. And for more than two decades, a treatment known as deep-brain stimulation (DBS) has been used to safely and effectively manage some symptoms of Parkinson’s disease, a brain disorder that can cause balance problems, tremors and slowed movements. It affects an estimated 1 million U.S. adults, most of whom are 60 and older.
To date, more than 200,000 DBS implants worldwide have been placed within patients to treat Parkinson’s and other disorders. Here’s how it works: A surgeon implants a thin wire, called a lead, into a specific region of the brain that, in Parkinson’s patients, produces irregular signals that cause tremor and other movement problems. The lead’s tip contains several metal wires called electrodes that can send out electrical pulses more than 100 times per second into the surrounding nerve cells in the brain. These pulses can drown out the problematic brain signals like a white-noise machine, helping to quell tremors and other movement symptoms.
For Florida resident Bruce Ryan, the idea of lodging something in his brain to help tame his tremors seemed “crazy” at first. “I just couldn’t imagine that happening to me,” says Ryan, 61. But his tremors were interfering with daily life. He didn’t want to go out to eat for fear of spilling something, and he couldn’t easily sign his name. So, he had the procedure.
Now, roughly five years later, Ryan said the difference it’s made in his life has been “profound.”
“It’s hard to put in words, but it’s one of the best decisions I’ve ever made in my life,” he says.
Though DBS is now a mature technology, researchers are still trying to improve it. For example, DBS pulse generators — devices implanted beneath the patient’s skin near the chest that send electrical signals to the wires in the brain — are getting smaller. And the batteries that power them, which once had to be changed every three to five years, are now rechargeable, lasting much longer.
Their controllability is also improving. Updated designs allow doctors to fine-tune the pulses to maximize their therapeutic benefit and minimize any potential side effects, such as slurring of speech that some people experience from the pulses interfering with healthy brain signaling.
A challenging road to clinical use
Experts stress that advanced BCIs face a long, winding road of tests and trials before they will become clinically available — and that as extraordinary as the technology’s potential is, it won’t be a cure-all.
For one, even the most advanced brain implant will yield minimal benefit if it’s not implanted in precisely the right spot, requiring a highly skilled team of specialists. “The difference between here to California, in brain-space, can be less than a millimeter,” says Michael Okun, the director of the University of Florida’s Norman Fixel Institute for Neurological Diseases and medical adviser for the Parkinson’s Foundation.
Patient-selection criteria are also key when it comes to these technologies. For example, not every Parkinson’s patient will benefit from DBS — in fact, only about 10 to 15 percent of people with Parkinson’s disease are considered good candidates for the treatment, and fewer still receive it. And while DBS can help with some symptoms of Parkinson’s, it doesn’t address all of them — nor does it stop or slow the disease’s underlying progression.
Crucially, experts say that BCIs are best seen as just one part of a broader care approach. In the NeuroRestore trials, for example, technology is only part of the equation; often the most important part is physical therapy. “I think a lot of people think technology alone is solving things,” Moraud says. “Technology is allowing physical therapy to work better.”
BCIs are still so new that physicians, device manufacturers and government regulators alike still have a lot of details to sort out.
“Who’s going to be part of that care team [for a BCI implantation]? How is that care delivered? How is it paid for?” McMullen asks. “All those questions need to be answered so that when one — or hopefully several — new BCIs go through the process and demonstrate they’re safe and effective, they can actually get to patients and have an impact.”
Michael Greshko is a journalist who has covered science and technology for publications including The New York Times, Science, Scientific American, and National Geographic, where he worked for seven years as a staff writer. He lives in Washington, D.C., with his family.




































































More From AARP
3 Brain-Stimulating Therapies for Depression That Can Work
ECT, TMS and vagus nerve stimulation are making a difference for some patientsNerve Stimulation for Stroke Recovery
Improvements in therapies and testing for Parkinson's and Alzheimer's
Smartwatches Keep Tabs on Health, Still Track Fitness
Features can keep you heart-healthy, give peace of mind