AARP Hearing Center

Freda Pyles’ road trip from Wichita, Kansas, to her home in Pennsylvania was a nightmare. Severe diarrhea meant urgent bathroom breaks at every rest stop for hundreds of miles. In Ohio, her husband took her to an emergency room. “I was dehydrated. My kidneys were shutting down,” says Pyles, 76, of her 2021 ordeal. “I couldn’t walk.”
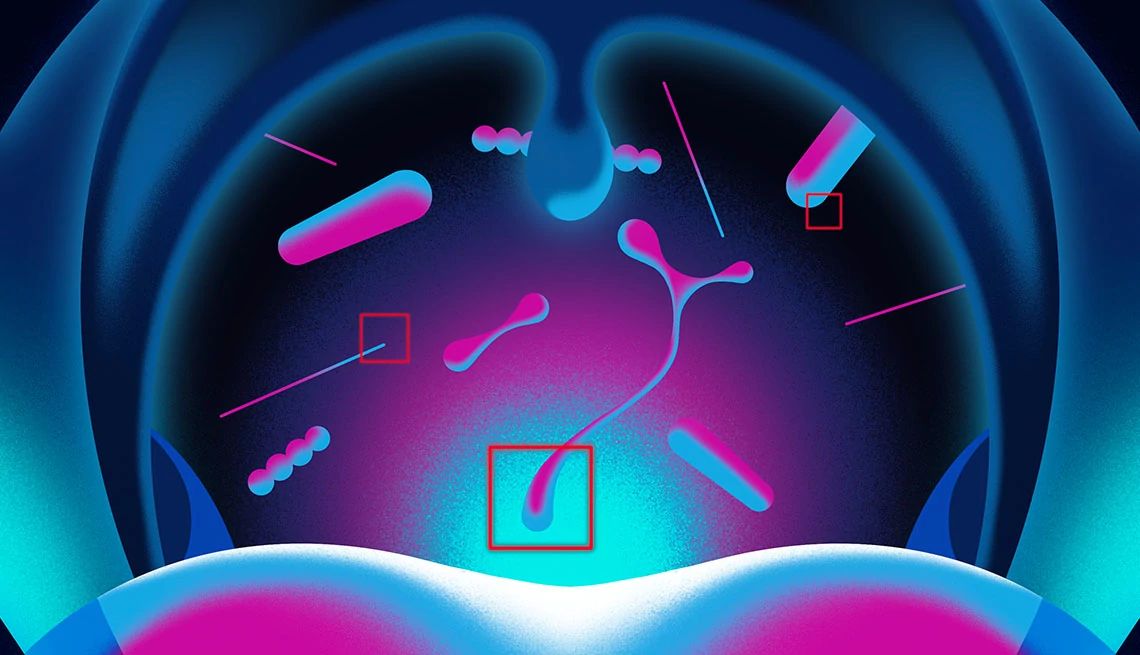
Pyles was infected with Clostridioides difficile (commonly known as C. diff or C. difficile) — a highly contagious germ that kills 30,000 Americans annually, most of them older adults. The infection took hold when an antibiotic prescribed for a tooth infection killed beneficial bacteria in her intestines that can normally keep C. diff in check.
A gold standard C. diff treatment, the antibiotic vancomycin, brought her a little relief but quickly set the stage for more trouble. It further wiped out protective gut bacteria, allowing tough C. diff spores to grow, release diarrhea-triggering toxins and kick-start a new infection.
Back home in rural Russell, Pennsylvania, Pyles spent her days in a chair close to the bathroom. She stopped gardening, keeping bees, swimming at the local YMCA and helping her husband take care of the couple’s flock of chickens. For four months she had recurrent C. diff infections. It’s a dangerous cycle that affects about 35 percent of older adults after a first bout of C. diff and kills 1 in 4 of them, according to a 2022 Yale University study of Medicare beneficiaries.
“By January I had lost 45 pounds, could hardly walk to the bathroom and had fallen a couple of times,” she says. “My husband was really worried. He told a friend he was slowly watching me die.”































































You Might Also Like
5 Easy Ways to Improve Your Gut Health
The 100 trillion microbes in your belly can keep you happy and healthy if you feed them right.The Diet That Helps IBS
Foods that hurt and foods that help25 Great Ways to Build Healthy Habits
These tips can help create changes that positively impact your well-being for years to comeRecommended for You